Impermissible Hindsight Leads to Reversal of Obviousness Finding
Attorney:
Derek Lightner, Ph.D.
April 24, 2025
In the appeal of USSN 17/963,407 (Appeal 2025-001881) to the Patent Trial and Appeal Board (PTAB), a finding of obviousness by Primary Examiner Drew Becker, supported by Supervisory Patent Examiner (SPE) Erik Kashnikow and Review Quality Assurance Specialist (RQAS) Jennifer McNeil from the Office of Patent Quality Assurance (OPQA), was reversed by the PTAB for the improper reliance on hindsight. The decision can be found at https://patentcenter.uspto.gov/applications/17963407/ifw/docs?application=.<... Read more
A Transformation of Matter Helps Secure Subject Matter Eligibility at the PTAB
Attorney:
Derek Lightner, Ph.D.
December 30, 2024
In the matter Ex parte MICHAEL J. WEST, in Technology Center 1700 (USSN 16/078,845; Appeal 2023-003903) rejections for an alleged lack of patentable subject matter, a lack of enablement, indefiniteness, and obviousness by a primary examiner, Lore Jarrett, were reversed by a panel of the Patent Trial and Appeal Board (PTAB) made up of Jeffrey R. Snay, Whitney Wilson, and Jane E. Inglese. The first appealed claim recited (with the underlined portions added after the first rejection): <... Read more
Missing Feature Overcomes Obviousness but Cautions Succinct Claiming and Argument
Attorney:
Derek Lightner, Ph.D.
October 7, 2024
On September 25, 2024, in Ex parte Sim, the Patent Trial and Appeal Board (PTAB) overturned the obviousness position of primary examiner, John Chu, supported by Supervisory Patent Examiners Mark Huff and Christine Tierney, in US Appl. No. 16/176,245 (Appeal 2023-003254, Technology Center 1700). Ex parte Sim involved a disagreement on the meaning of recitation regarding a copolymer structure.<... Read more
PTAB Finds Method Involving "Growing, Selecting, and Crossing" Sufficient for Integrating Genome Estimation Data Set Into Practical Application
Attorney:
Nicholas Rosa, Ph.D.
September 17, 2024
The Patent Trial and Appeal Board (PTAB) recently reversed a final rejection based on § 101 by finding that a method for selecting individuals for a breeding program that recited the steps of “growing, selecting, and crossing” did integrate an “optimized [genome] estimation data set” judicial exception into practical application. These actionable steps and the distinct advantages the method represented over conventional breeding methods involving other types of genomic prediction described in the specification were instrumental in the PTAB’s findings. The inclusion of such actionable steps and description of the advantages of the subject matter of the application may be useful to support amendments that may be necessary to overcome § 101 rejections.<... Read more
USPTO Seeks Public Feedback on The Experimental Use Exception to Patent Infringement
Attorney:
Evan Smith
July 26, 2024
On June 27, 2024, the United States Patent and Trade Office (“USPTO”) published a request for comments (“RFC”) in the Federal Register inquiring about the current state of the experimental use defense to patent infringement and to determine the potential value of legislative action on the issue. This RFC both supports the President’s 2021 Executive Order on Promoting Competition in the American Economy and furthers the United States Department of Agriculture and the USPTO’s joint commitment to evaluate “New proposals for incentivizing and protecting innovation in the seed and agricultural-related space, including the addition of research or breeders' exemptions for U.S. utility patents.”[1]<... Read more
PTAB Reverses Examiners on Unexpected Results
Attorney:
Derek Lightner, Ph.D.
June 11, 2024
In Ex parte Freeman (USSN 16/270,259; TC 1600; Appeal 2023-000512, the underlying application being referred to herein as the “Freeman application”), a finding of obviousness and obviousness-type double patenting (ODP) was reversed on May 24, 2024. Freeman’s application was examined by Devang Thakor initially, but the case was taken over by another primary examiner, Nicole Babson, whose position was supported by Supervisor Patent Examiners, David Blanchard and Bethany Barham.<... Read more
PTAB Finds Non-Obviousness for Missing Element
Attorney:
Derek Lightner, Ph.D.
February 15, 2024
The Patent Trial and Appeal Board (PTAB) recently reversed the novelty, obviousness, and obviousness-type double patenting (ODP) rejections of the examining corps in a Track One case (USSN 17/342,945; Appeal 2023-004168; TC 1600). Led by Administrative Patent Judge (APJ) Richard Lebovitz, the PTAB panel further including APJs John E. Scheider and Eric B. Grimes reversed the rejections of Examiner Jake M. Vu, whose position was supported by Supervisory Patent Examiner (SPE) Michael G. Hartley, quality control SPE Scarlett Goon, and SPE Frederick F. Krass.<... Read more
USPTO Releases Examiner Guidance for The Amgen Enablement Decision
Attorney:
Richard D. Kelly
January 10, 2024
The USPTO today published its guidance to the examiners on the impact of the Amgen v. Sanofi,143 S. Ct. 1243 (2023), on USPTO practice. The Guidance is basically steady as she goes but with the caveat that the enablement requirement and the Wands factors (In re Wands, 858 F.2d 731, 737 (Fed. Cir. 1988)) apply across all technologies, noting the Court’s reliance on cases involving the telegraph, incandescent lamp filaments and wood glue. The Guidance also noted that the post-Amgen Federal Circuit decisions had reaffirmed the continued validity of the Wands factors as consistent with Amgen, citingMedytox, 71 F.4th at 998- 999, Baxalta Inc, v. Genentech, Inc., 81 F.4th 1362 (Fed. Cir. 2023), and In re Starrett, 2023 WL 3881360 (Fed. Cir. 2023) (non-precedential).<... Read more
PTAB Overturns Obviousness Finding for Intended Use-Functional Characteristic Error
Attorney:
Derek Lightner, Ph.D.
October 10, 2023
On October 3, 2023, the Patent Trial and Appeal Board (PTAB) reversed an examiner’s finding of obviousness based (i) on mischaracterizing a claim feature as an intended use, the feature instead being considered by the PTAB as a characteristic of the composition of the claimed article, and (ii) extrapolating a trend in the cited art without explicit support for the extrapolation. The appeal (No. 2022-003575, USSN 13/375,167, Technology Center 1700) of Ex parte YOSHIMITSU ODA and MASAAKI ISHIO was centered on an independent claim, reciting:<... Read more
In Re Cellect – ODP Defense Does Not Impact The Expiration Date of A Patent With Both PTA and PTE In The Absence of A Terminal Disclaimer
Attorney:
Richard D. Kelly
September 15, 2023
The Federal Circuit in In re Cellect, Appeals Nos. 2022-1293, 2022-1294, 2022-1295, 2022-1296 held that the earliest patent to expire in a series of patents subject to obviousness-type double patenting (ODP) controls, i.e., the PTA in the later to expire patents is lost and all patents are invalid for double patenting. In Cellect the relation between the patents is shown below:
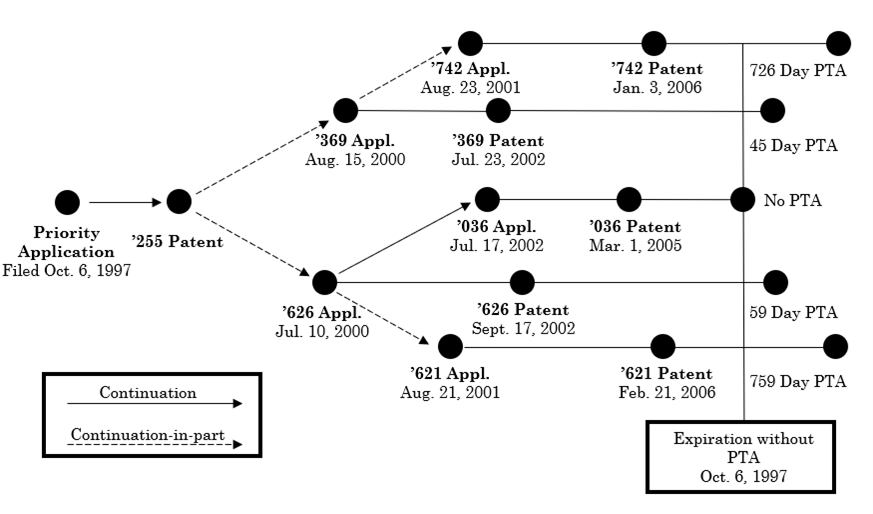 <... Read more
<... Read more