The Muted Response of Pharmaceutical Companies to the Establishment of the Unitary Patent Court
Attorney:
Matthew Hampton
March 3, 2025
The Unified Patent Court (UPC) is an international court set up by participating European Union Member States to deal with the infringement and validity of both Unitary Patents1 and European patents. The Agreement on a Unified Patent Court (UPCA) entered into force on 1st June 2023 with the intention of obviating costly parallel patent litigation in the participating European Union Member States and thereby enhancing legal certainty for rights holders.<... Read more
Federal Circuit Reverses District Court's Invalidity Ruling on Written Description in Novartis v. Torrent
Attorney:
Xiaohua (Joyce) Guo, Ph.D.
February 18, 2025
In a precedential opinion issued on January 10, 2025, the United States Court of Appeals for the Federal Circuit reversed a district court’s ruling that had invalidated claims 1-4 of U.S. Patent No. 8,101,659 (“the ’659 patent”) for lack of written description.<... Read more
PTAB Reverses Examiner Based on "Or" Language
Attorney:
Diane Jones
February 14, 2025
Re: US2016/0331260A1 “Asystole detection for cardiopulmonary resuscitation”, Applicant Koninklijke Philips N.V.<... Read more
Patenting of Multi-Disciplinary Subject Matter – Personalized Medicine
Attorney:
Robert W. Downs
February 7, 2025
Personalized medicine is especially intriguing, as a problem with most drugs are the potential side effects. From a naive perspective, administering drugs may appear to be a trial and error process: take this for two weeks and come back with a follow up visit. If you have any side effects, contact me immediately. Wouldn’t it be nice if a drug had the effect it was meant to have without the possibility of side effects?<... Read more
The CAFC Backs the FTC View on Listing Device Patents in the Orange Book
Attorney:
Richard D. Kelly
January 14, 2025
On December 20 the Federal Circuit in Teva Branded Pharmaceutical Products R&D, Inc. (Teva) v. Amneal Pharmaceuticals (Amneal) affirmed the district court’s decision that medical devices were not listable in the Orange Book and Teva had improperly listed patents in the Orange Book and required Teva to remove them.<... Read more
A Transformation of Matter Helps Secure Subject Matter Eligibility at the PTAB
Attorney:
Derek Lightner, Ph.D.
December 30, 2024
In the matter Ex parte MICHAEL J. WEST, in Technology Center 1700 (USSN 16/078,845; Appeal 2023-003903) rejections for an alleged lack of patentable subject matter, a lack of enablement, indefiniteness, and obviousness by a primary examiner, Lore Jarrett, were reversed by a panel of the Patent Trial and Appeal Board (PTAB) made up of Jeffrey R. Snay, Whitney Wilson, and Jane E. Inglese. The first appealed claim recited (with the underlined portions added after the first rejection): <... Read more
Missing Feature Overcomes Obviousness but Cautions Succinct Claiming and Argument
Attorney:
Derek Lightner, Ph.D.
October 7, 2024
On September 25, 2024, in Ex parte Sim, the Patent Trial and Appeal Board (PTAB) overturned the obviousness position of primary examiner, John Chu, supported by Supervisory Patent Examiners Mark Huff and Christine Tierney, in US Appl. No. 16/176,245 (Appeal 2023-003254, Technology Center 1700). Ex parte Sim involved a disagreement on the meaning of recitation regarding a copolymer structure.<... Read more
Myrbetriq® Patent Invalidated On Grounds Not Asserted by Any Party Violating Party Presentation Principle
Attorney:
Richard D. Kelly
October 2, 2024
In Astellas Pharma Inc. v. Sandoz Inc,, et al, following a five-day patent claims bench trial the court found asserted claims 5, 20, and 25 invalid under 35 U.S.C. § 101 even though Sandoz had not asserted 101 as defense at any time during the case. The parties had agreed to limit the issue to claims 5, 20, and 25 and the defense to invalidity under 35 U.S.C. § 112. Neither party had notice that patent eligibility was an issue. In making his ruling Judge Bataillon relied upon a statement in Astellas post-trial brief that the “inventive concept of the ’780 Patent was discovering the dissolution rate that would address the food effect and achieving it using previously known formulation technology.” Thus, because the claimed invention “reflects merely the discovery of the food-effect-resolving dissolution profile,” the district court deemed the asserted claims invalid as patent ineligible. Not only had the issue of patent eligibility not been raised by the defendant, but the claims were also to a specific composition. Recognizing the district court had gone on astray Sandoz moved the court to make additional findings of fact advising the court that the § 101 defense had not been raised as a defense and that the decision must be based on the language in the claims. The motion was denied.<... Read more
PTAB Finds Method Involving "Growing, Selecting, and Crossing" Sufficient for Integrating Genome Estimation Data Set Into Practical Application
Attorney:
Nicholas Rosa, Ph.D.
September 17, 2024
The Patent Trial and Appeal Board (PTAB) recently reversed a final rejection based on § 101 by finding that a method for selecting individuals for a breeding program that recited the steps of “growing, selecting, and crossing” did integrate an “optimized [genome] estimation data set” judicial exception into practical application. These actionable steps and the distinct advantages the method represented over conventional breeding methods involving other types of genomic prediction described in the specification were instrumental in the PTAB’s findings. The inclusion of such actionable steps and description of the advantages of the subject matter of the application may be useful to support amendments that may be necessary to overcome § 101 rejections.<... Read more
Finally, an ODP Decision in Favor of the Patentee
Attorney:
Richard D. Kelly
August 28, 2024
In Allergan USA, Inc v Sun Pharmaceutical Indus. Ltd, Appeal No. 2024-1061, August 13, 2024, the Federal Circuit reversed the district court’s holding that Allergan patent, U.S.P. 7,741,356 (‘356) was invalid for obviousness-type double patenting over USPs 11,007,179, 11,090,291, and 11,311,516. The relationship between the patents is shown below:
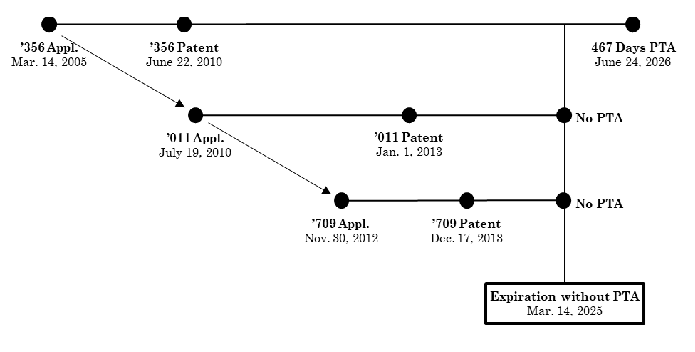
The ‘356 patent was entitled to significant PTA but because of the PTE it received, Allergan effectively disclaimed all but 487 days of the PTA. The total extension PTE plus PTA resulted in an expiration date of May 27, 2029, 15 years from the ‘356 issue date -- the maximum extension possible.<... Read more