PTAB Finds Non-Obviousness for Missing Element
February 15, 2024
The Patent Trial and Appeal Board (PTAB) recently reversed the novelty, obviousness, and obviousness-type double patenting (ODP) rejections of the examining corps in a Track One case (USSN 17/342,945; Appeal 2023-004168; TC 1600). Led by Administrative Patent Judge (APJ) Richard Lebovitz, the PTAB panel further including APJs John E. Scheider and Eric B. Grimes reversed the rejections of Examiner Jake M. Vu, whose position was supported by Supervisory Patent Examiner (SPE) Michael G. Hartley, quality control SPE Scarlett Goon, and SPE Frederick F. Krass.<... Read more
Written Description of a Numerical Range
February 14, 2024
The Federal Circuit on February 9 decided another written description case involving a range not found in ipsis verbis in the patent specification in RAI Strategic Holdings Inc. v. Philip Morris Products S.A. RAI appealed from a PTAB holding in PGR2020-00071 that claims 10 and 27 of U.S.P. 10,492,542 lacked written description in the specification. Claims 27 is reproduced below; claim 10 is identical except it depends on claim 9:<... Read more
When does a drug label induce infringement under 35 U.S.C. § 271(e)(2) of a patent not claiming an indication or method of use?
January 23, 2024
In Corcept Therapeutics, Inc. v. Teva Pharmaceuticals U.S.A., Inc., C.A. 18-03632, December 29, 2023, the Chief Judge Bumb of the New Jersey District Court wrestled with the question of infringement under 35 U.S.C. 271(e)(2) where the drug labels of the generic and ethical drug were identical in the relevant parts.<... Read more
Invalidity of a Patent Claiming Antibodies Characterized by Their Function In View of Amgen
January 12, 2024
Baxalta Inc. and Baxalta GmbH (Baxalta) appealed a district court decision that the claims of U.S. Patent No. 7,033,590 were invalid for lack of enablement. The Federal Circuit (the Court) affirmed the decision.<... Read more
USPTO Releases Examiner Guidance for The Amgen Enablement Decision
January 10, 2024
The USPTO today published its guidance to the examiners on the impact of the Amgen v. Sanofi,143 S. Ct. 1243 (2023), on USPTO practice. The Guidance is basically steady as she goes but with the caveat that the enablement requirement and the Wands factors (In re Wands, 858 F.2d 731, 737 (Fed. Cir. 1988)) apply across all technologies, noting the Court’s reliance on cases involving the telegraph, incandescent lamp filaments and wood glue. The Guidance also noted that the post-Amgen Federal Circuit decisions had reaffirmed the continued validity of the Wands factors as consistent with Amgen, citingMedytox, 71 F.4th at 998- 999, Baxalta Inc, v. Genentech, Inc., 81 F.4th 1362 (Fed. Cir. 2023), and In re Starrett, 2023 WL 3881360 (Fed. Cir. 2023) (non-precedential).<... Read more
In Patent Eligibility It's the Claim That Is the Name of the Game*
December 18, 2023
Recently Judge Connolly, Chief Judge of the District Court of Delaware had an opportunity of deciding three motions for summary judgment filed by CareDX in its litigation with Natera, Natera, Inc. v. CareDX, Inc, Dist. DE, CA 20-38, that one patent was invalid as being directed to patent ineligible subject matter. The patents are U.S. Patent Nos. 10,597,724 (‘724), 10,655,180 (‘180), and 11,111,544 (‘544). The ‘724 and ‘180 patents are directed to methods of observing DNA in samples taken from patients. The ‘544 patent is directed to a method of “preparing a preparation of amplified DNA” from the sample of an individual to observe the DNA of a second individual in the sample. The Court found that one claim in each patent was representative of all claims in the respective patent. These representative claims are:<... Read more
Generics Beat the Tax Man
November 27, 2023
Recently Mylan in Mylan, Inc. v. Comm’r of Internal Revenue, 76 F4th 230 (3rd Cir. 2023), beat the tax man. Mylan tried to deduct its ANDA litigation expenses as ordinary and necessary business expenses which are an immediate deduction, while the Internal Revenue Service (IRS) claimed the expenses should be capitalized which is not as valuable. The IRS asserted that the expenses should be capitalized, under 26 U.S.C. § 263 and the associated regulations, similar to money paid to acquire approvals from the Food and Drug Administration (“FDA”). Mylan at 243. The Court rejected the IRS’ argument observing “ultimate FDA approval is never decided by the outcome of patent litigation under [35 U.S.C.] § 271(e)(2), even if it is delayed by such litigation.” Mylan at 244. The Court concluded “it makes no difference in deciding the question of deductibility whether the patent litigation expenses are incurred by the patentee or the alleged infringer. Nor does it matter that the deductibility question arises in the context of an ANDA suit.” Mylan at 239-40.<... Read more
Sisvel – PTAB Guidance On A Proposed Amendment In An IPR Is Not Binding On The PTAB
November 20, 2023
In Sisvel Int’l S.A. v. Sierra Wireless, Inc., Appeal nos. 2022-1387 and 2022-1492, (Fed. Cir. 2023) the Court considered this issue of when was a claim broadened in a post grant proceeding. Sisvel’s patent U.S. 7,433,698 (‘698) was involved in two IPRs, IPR 2020-01070 and IPR2020-01071 where it attempted unsuccessfully to amend its claim 10. Sisvel received preliminary guidance from the PTAB on its original motion to amend claim 10 which then led it to file a revised motion to amend. Sisvel’s revised motion proposed these amendments:
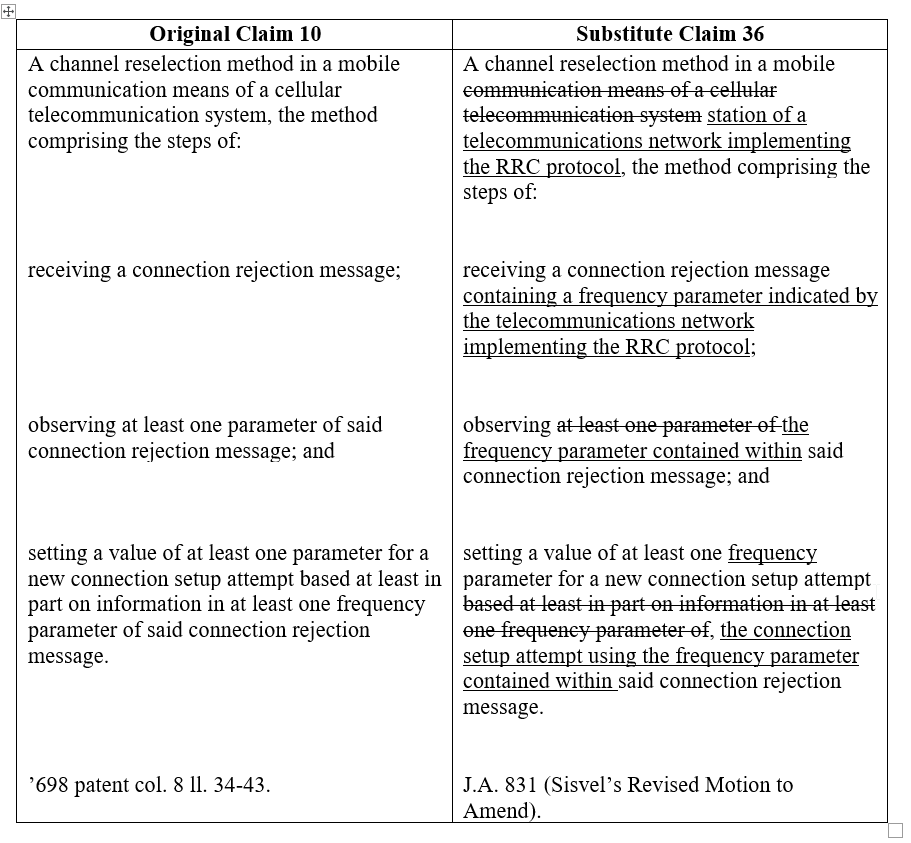
The amendments to the preamble and the first two limitations result in a more limited claim than original claim 10. However, the PTAB and Federal Circuit held the third limitation “setting the value” to be broader than the original limitation. The original claim required that the value of at least one parameter be based at least in part on information in the at least one frequency parameter. The amended claim required the “use of the frequency parameter” which was interpreted as being broader in scope than the “based on” language of the original claim 10. Using the information was broader in scope than the old language “based at least in part on information in at least one frequency parameter.” “Using the parameter” was broader than basing it on the parameter. The Federal Circuit used the example of using a value V and multiplied by X and then dividing by X where the value X was used to calculate V, but the value V is not based on X. Thus, while proposed claim 36 is narrower overall than claim 10, the last limitation is broader. It is possible for infringement of claim 36 to exist where none existed for claim 10 since a device only “using the frequency parameter” of claim 36 would not infringe claim 10 but would infringe proposed claim 36. The amendment process does not permit a patentee to broaden any aspect of a claim even though the overall the claim is narrower, see Hockerson-Halberstadt, 183 F.3d at 1374 see also 37 C.F.R. § 1.175(b) (“A claim is a broadened claim if the claim is broadened in any respect.”)<... Read more
FTC Alleges Misuse of Orange Book Listings: Puts10 Pharma Companies on Notice
November 10, 2023
On Tuesday, the FTC announced that it is cracking down on companies it asserts is improperly or inaccurately placing drugs on the list of FDA-approved products, known as the Orange Book. The challenge may result in removal of products from the Orange Book.<... Read more
FDA Announces Formation of a New Digital Health Advisory Committee
October 19, 2023
On October 11, the FDA announced the formation of a new Digital Health Advisory Committee to assist the FDA in exploring the complex, scientific and technical issues related to digital health technologies (DHTs). The committee’s function is to advise the FDA on issues related to DHTs, help the agency understand the benefits, risks, and clinical outcomes associated with use of DHTs. The FDA notice explained that:<... Read more