PTAB Overturns Obviousness Finding for Intended Use-Functional Characteristic Error
October 10, 2023
On October 3, 2023, the Patent Trial and Appeal Board (PTAB) reversed an examiner’s finding of obviousness based (i) on mischaracterizing a claim feature as an intended use, the feature instead being considered by the PTAB as a characteristic of the composition of the claimed article, and (ii) extrapolating a trend in the cited art without explicit support for the extrapolation. The appeal (No. 2022-003575, USSN 13/375,167, Technology Center 1700) of Ex parte YOSHIMITSU ODA and MASAAKI ISHIO was centered on an independent claim, reciting:<... Read more
The Federal Circuit's First Enablement Decision Since Amgen
September 25, 2023
Federal Circuit applied Amgen v. Sanofi, 598 U.S. 594 (2013)in deciding that claims 1-4, 19 and 20 of Baxalta’s hemophilia patent, U.S. Patent 7,033,590 (‘590), were invalid as lacking an enabling disclosure.<... Read more
In Re Cellect – ODP Defense Does Not Impact The Expiration Date of A Patent With Both PTA and PTE In The Absence of A Terminal Disclaimer
September 15, 2023
The Federal Circuit in In re Cellect, Appeals Nos. 2022-1293, 2022-1294, 2022-1295, 2022-1296 held that the earliest patent to expire in a series of patents subject to obviousness-type double patenting (ODP) controls, i.e., the PTA in the later to expire patents is lost and all patents are invalid for double patenting. In Cellect the relation between the patents is shown below:
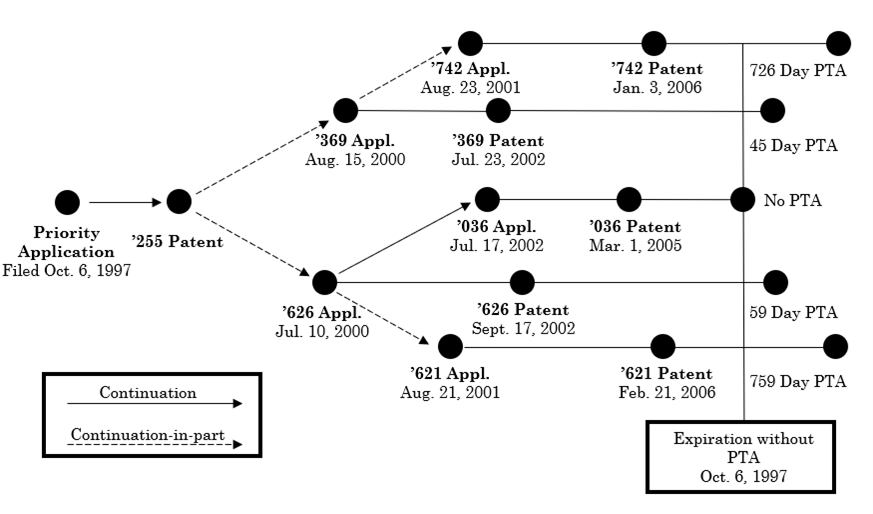 <... Read more
<... Read more
Federal Circuit Determines Anticipation of Dependent Claims and Sufficient Evidence of Commercial Success
September 6, 2023
The Court of Appeals for the Federal Circuit (CAFC), on August 16, 2023, affirmed the U.S. Patent and Trademark Office Patent Trial and Appeal Board’s (PTAB) decision, that the claims of Incept LLC’s U.S. Patent No. 8,257,723 (‘723) and U.S. Patent No. 7,744,913 (‘913) are unpatentable as being anticipated by, or obvious over asserted prior art. The decision on appeal considered whether the PTAB erred in its final decision that Palette Life Sciences, Inc. (Palette) had established the challenged claims to be unpatentable over prior art for the inter partes reviews of the ‘723 and ‘913 patents.<... Read more
Federal Circuit Reiterates What Constitutes A Motivation to Combine, A Reasonable Expectation of Success, and Unexpected Results in New Chemical Compounds
August 30, 2023
In Sun Pharmaceutical Industries, Inc. v. Incyte Corporation, on August 22, 2023, the Federal Circuit affirmed a Final Written Decision of the Patent and Trial Appeal Board (the Board) of an inter partes review (IPR) asserting the claims of U.S. Patent No. 9,249,149 (the ’149 patent) as obvious under 35 U.S.C. § 103. The central argument was whether Sun’s “octo-deuterated” ruxolitinib analog (CTP-543) and “tetra-deuterated” ruxolitinib analogs, arising from claim 7 of the ’149 patent, were obvious in light of the prior art references presented by Incyte (Rodgers, Shilling, and the Concert Backgrounder).
Claim 7 recited:
The compound of claim 1, in which the compound is selected from the group consisting of:
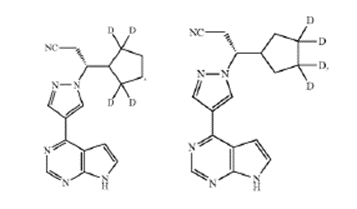
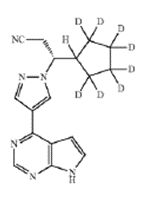
or a pharmaceutically acceptable salt of any of the foregoing.<... Read more
No Reasonable Expectation of Success in Modifying Non-Overlapping Range to Overlap
August 28, 2023
In the matter of Ex parte HAN LIU, et al., the Patent Trial and Appeal Board (PTAB) determined on August 17, 2023, that an examiner (Sarah al-Awadi, supported by Supervisory Patent Examiners, David J. Blanchard and Sue X. Liu) failed to establish the prima facie obviousness of claims by failing to establish a clear motivation and a reasonable expectation of success in maintaining the function of a copolymer-Ag system, inter alia, in modifying a particular monomer content to overlap with the claims against the disclosure of the primary references. The independent claims in question recited (emphasis added):<... Read more
American Axle Part 2 – Not an Abstract Idea
August 4, 2023
On July 28, 2023, Judge Williams of the Delaware District unsealed his decision on remand from the Federal Circuit addressing competing summary judgment motions regarding the patent eligibility of remanded claim 1. The Federal Circuit remanded the case to the district court to determine if claim 1 was directed to an abstract idea argued by Neapco on appeal for invalidity. Claim 1 provided:<... Read more
USPTO Announces Revised Director Review Process
July 26, 2023
On July 24, the USPTO announced a revised Director interim review process. Although the USPTO in July 2022 had requested comments on its director review process and the comment period was closed on October 19, 2022, the USPTO has still not formalized the process or published any proposed rules to implement it. Instead, it revised its interim review process.<... Read more
No Motivation to Modify Product-Specific Method with Method Features for Different Products
July 14, 2023
On July 10, 2023, the Patent Trial and Appeal Board (PTAB) reversed an examiner’s finding of obvious on the basis of a failure to show a motivation to modify the prior art and a lack of a reasonable expectation of success. The appeal (No. 2023-002080, USSN 15/558,153, Technology Center 1700) of Ex parte MARK HETHERINGTON began with the filing of a Notice of Appeal on May 13, 2022, after filing the national stage application on September 13, 2017. The main appealed claim recited:<... Read more
The Federal Circuit Takes Design Patent Obviousness En Banc
July 11, 2023
The Federal Circuit on June 30 granted a petition for re-hearing en banc of its per curiam decision in LKQ Corp. v. GM Global Tch. Operations, LLC. finding the PTAB had correctly decided that GM’s design patent D797,625 was not unpatentable, i.e., valid. The issue raised was whether the Federal Circuit’s rulings in Durling v. Spectrum Furniture Co., Inc., 101 F.3d 100 (Fed. Cir. 1996); In re Rosen, 673 F.2d 388 in (C.C.P.A. 1982) followed by the PTAB in its decision created a “rigid” rule for obviousness in design patent in violation of Supreme Court’s 2007 KSR decision overturning the Federal Circuit’s rigid rule for obviousness of utility patents, the “teaching, suggestion motivation“ (TSM) test for obviousness. <... Read more