US Synthetic Corp. v. International Trade Commission: Safeguarding Structure and Property Limitations in Composition-of-Matter Claims
Attorney:
David Inglefield
March 10, 2025
On February 13, the U.S. Court of Appeals for the Federal Circuit (CAFC) reversed the decision of the International Trade Commission (ITC) in US Synthetic Corp. v. International Trade Commission, issuing a precedential decision regarding subject matter eligibility important to the pharmaceutical and other life science industries. The ITC had previously ruled that US Synthetic’s composition of matter claims reciting measured properties of a composition were directed to a patent-ineligible abstract idea under 35 U.S.C. § 101. The CAFC found that the claims were indeed related to concrete structures, not patent-ineligible abstract ideas, and affirmed the lower court’s finding regarding enablement.<... Read more
The Muted Response of Pharmaceutical Companies to the Establishment of the Unitary Patent Court
Attorney:
Matthew Hampton
March 3, 2025
The Unified Patent Court (UPC) is an international court set up by participating European Union Member States to deal with the infringement and validity of both Unitary Patents1 and European patents. The Agreement on a Unified Patent Court (UPCA) entered into force on 1st June 2023 with the intention of obviating costly parallel patent litigation in the participating European Union Member States and thereby enhancing legal certainty for rights holders.<... Read more
Federal Circuit Reverses District Court's Invalidity Ruling on Written Description in Novartis v. Torrent
Attorney:
Xiaohua (Joyce) Guo, Ph.D.
February 18, 2025
In a precedential opinion issued on January 10, 2025, the United States Court of Appeals for the Federal Circuit reversed a district court’s ruling that had invalidated claims 1-4 of U.S. Patent No. 8,101,659 (“the ’659 patent”) for lack of written description.<... Read more
Patenting of Multi-Disciplinary Subject Matter – Personalized Medicine
Attorney:
Robert W. Downs
February 7, 2025
Personalized medicine is especially intriguing, as a problem with most drugs are the potential side effects. From a naive perspective, administering drugs may appear to be a trial and error process: take this for two weeks and come back with a follow up visit. If you have any side effects, contact me immediately. Wouldn’t it be nice if a drug had the effect it was meant to have without the possibility of side effects?<... Read more
The CAFC Backs the FTC View on Listing Device Patents in the Orange Book
Attorney:
Richard D. Kelly
January 14, 2025
On December 20 the Federal Circuit in Teva Branded Pharmaceutical Products R&D, Inc. (Teva) v. Amneal Pharmaceuticals (Amneal) affirmed the district court’s decision that medical devices were not listable in the Orange Book and Teva had improperly listed patents in the Orange Book and required Teva to remove them.<... Read more
Myrbetriq® Patent Invalidated On Grounds Not Asserted by Any Party Violating Party Presentation Principle
Attorney:
Richard D. Kelly
October 2, 2024
In Astellas Pharma Inc. v. Sandoz Inc,, et al, following a five-day patent claims bench trial the court found asserted claims 5, 20, and 25 invalid under 35 U.S.C. § 101 even though Sandoz had not asserted 101 as defense at any time during the case. The parties had agreed to limit the issue to claims 5, 20, and 25 and the defense to invalidity under 35 U.S.C. § 112. Neither party had notice that patent eligibility was an issue. In making his ruling Judge Bataillon relied upon a statement in Astellas post-trial brief that the “inventive concept of the ’780 Patent was discovering the dissolution rate that would address the food effect and achieving it using previously known formulation technology.” Thus, because the claimed invention “reflects merely the discovery of the food-effect-resolving dissolution profile,” the district court deemed the asserted claims invalid as patent ineligible. Not only had the issue of patent eligibility not been raised by the defendant, but the claims were also to a specific composition. Recognizing the district court had gone on astray Sandoz moved the court to make additional findings of fact advising the court that the § 101 defense had not been raised as a defense and that the decision must be based on the language in the claims. The motion was denied.<... Read more
Finally, an ODP Decision in Favor of the Patentee
Attorney:
Richard D. Kelly
August 28, 2024
In Allergan USA, Inc v Sun Pharmaceutical Indus. Ltd, Appeal No. 2024-1061, August 13, 2024, the Federal Circuit reversed the district court’s holding that Allergan patent, U.S.P. 7,741,356 (‘356) was invalid for obviousness-type double patenting over USPs 11,007,179, 11,090,291, and 11,311,516. The relationship between the patents is shown below:
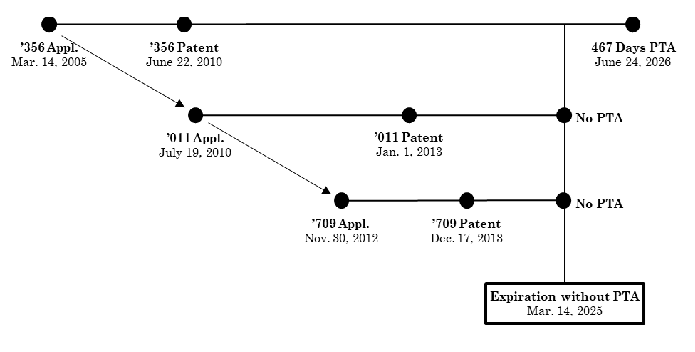
The ‘356 patent was entitled to significant PTA but because of the PTE it received, Allergan effectively disclaimed all but 487 days of the PTA. The total extension PTE plus PTA resulted in an expiration date of May 27, 2029, 15 years from the ‘356 issue date -- the maximum extension possible.<... Read more
USPTO Seeks Public Feedback on The Experimental Use Exception to Patent Infringement
Attorney:
Evan Smith
July 26, 2024
On June 27, 2024, the United States Patent and Trade Office (“USPTO”) published a request for comments (“RFC”) in the Federal Register inquiring about the current state of the experimental use defense to patent infringement and to determine the potential value of legislative action on the issue. This RFC both supports the President’s 2021 Executive Order on Promoting Competition in the American Economy and furthers the United States Department of Agriculture and the USPTO’s joint commitment to evaluate “New proposals for incentivizing and protecting innovation in the seed and agricultural-related space, including the addition of research or breeders' exemptions for U.S. utility patents.”[1]<... Read more
PTAB Invalidates Regeneron Claims on Method of Treatment
Attorney:
Cristina Lai
July 5, 2024
The Patent Trial and Appeal Board (PTAB) issued a final written decision on IPR2023-00442 determining that claims 1, 3-11, 13, 14, 16-24, and 26 of U.S. Patent No. 10,130,681 (“the ‘681 patent”) were unpatentable. The ‘681 patent is owned by Regeneron Pharmaceuticals, Inc., and was challenged in an IPR by Samsung Bioepis Co., Ltd. The ‘681 patent claims priority to a number of patents that were invalidated in previous IPR proceedings.<... Read more
PTAB Reverses Examiners on Unexpected Results
Attorney:
Derek Lightner, Ph.D.
June 11, 2024
In Ex parte Freeman (USSN 16/270,259; TC 1600; Appeal 2023-000512, the underlying application being referred to herein as the “Freeman application”), a finding of obviousness and obviousness-type double patenting (ODP) was reversed on May 24, 2024. Freeman’s application was examined by Devang Thakor initially, but the case was taken over by another primary examiner, Nicole Babson, whose position was supported by Supervisor Patent Examiners, David Blanchard and Bethany Barham.<... Read more