The Limits of Inherency in Product-by-Process Claims, and When is an Isolated Cell Actually a Population?
Attorney:
Lucas Koziol, Ph.D.
April 7, 2025
Restem, LLC, v. Jadi Cell, LLC (see the Resources link below for a copy of the Opinion)<... Read more
Prosecution Disclaimer in a Member of a Patent Family Over the Prosecution History of Another Member of the Family
Attorney:
Richard D. Kelly
March 27, 2025
In Maquet Cardiovascular LLC. v. Abiomed Inc., Appeal No. 2023-2045, March 21, 2025, the Federal Circuit provided guidance as to when the prosecution history of one member of a patent family may act as an estoppel in the claim construction of another member of the family. At issue was the construction of claims 1 and 24 of U.S.P. 10,238,783 (‘783) in view of the prosecution histories of its parent application, U.S.P. 9,789,238 (‘238) and great-great-grandparent application U.S.P. 8,888,728 (‘728). The ‘783 patent was directed to blood pumps which could be placed in a patient’s vascular system without using a supplemental guide means. The guide was integrated into the apparatus.<... Read more
PTAB Invalidates Regeneron Claims on Method of Treatment
Attorney:
Cristina Lai
July 5, 2024
The Patent Trial and Appeal Board (PTAB) issued a final written decision on IPR2023-00442 determining that claims 1, 3-11, 13, 14, 16-24, and 26 of U.S. Patent No. 10,130,681 (“the ‘681 patent”) were unpatentable. The ‘681 patent is owned by Regeneron Pharmaceuticals, Inc., and was challenged in an IPR by Samsung Bioepis Co., Ltd. The ‘681 patent claims priority to a number of patents that were invalidated in previous IPR proceedings.<... Read more
IOEngine v. Ingenico: Printed Matter Doctrine and Forfeiture of Claim Construction
Attorney:
Xiaohua (Joyce) Guo, Ph.D.
May 30, 2024
In a precedential decision, the US Court of Appeals for the Federal Circuit partially reversed and partially affirmed the Final Written Decisions made by the Patent Trial and Appeals Board (“Board”) during a series of inter partes review (IPR) proceedings.<... Read more
Federal Circuit Upholds PTAB Finding of Patentability in Medtronic v. Teleflex Life Sciences
April 1, 2024
In Medtronic, Inc. v. Teleflex Life Sciences Ltd. the Federal Circuit upheld the Patent Trial and Appeal Board (PTAB) decision that U.S. Patent No. 8,142,413 (“the ’413 patent”), owned by Teleflex, was not shown to be unpatentable over the asserted prior art. This post will focus on how the Federal Circuit affirmed the PTAB’s claim interpretation to find ’413 patent claims 1, 2, 4, 5, and 7–14 non-obvious.<... Read more
Federal Circuit Remands to PTAB to Further Consider Pfizer's Motion to Amend Claims
Attorney:
Long Phan
March 13, 2024
In a recent ruling regarding Pfizer's pneumococcal vaccine patent (U.S. Patent No. 9,492,559), the Federal Circuit upheld most of the Patent Trial and Appeal Board’s (PTAB) decision to invalidate portions of Pfizer's ‘559 patent as obvious. The PTAB’s invalidation of the ‘559 patent occurred over five IPRs by Merck Sharp & Dohme Corp., Sanofi Pasteur Inc. and SK Chemicals Co.<... Read more
Sisvel – PTAB Guidance On A Proposed Amendment In An IPR Is Not Binding On The PTAB
Attorney:
Richard D. Kelly
November 20, 2023
In Sisvel Int’l S.A. v. Sierra Wireless, Inc., Appeal nos. 2022-1387 and 2022-1492, (Fed. Cir. 2023) the Court considered this issue of when was a claim broadened in a post grant proceeding. Sisvel’s patent U.S. 7,433,698 (‘698) was involved in two IPRs, IPR 2020-01070 and IPR2020-01071 where it attempted unsuccessfully to amend its claim 10. Sisvel received preliminary guidance from the PTAB on its original motion to amend claim 10 which then led it to file a revised motion to amend. Sisvel’s revised motion proposed these amendments:
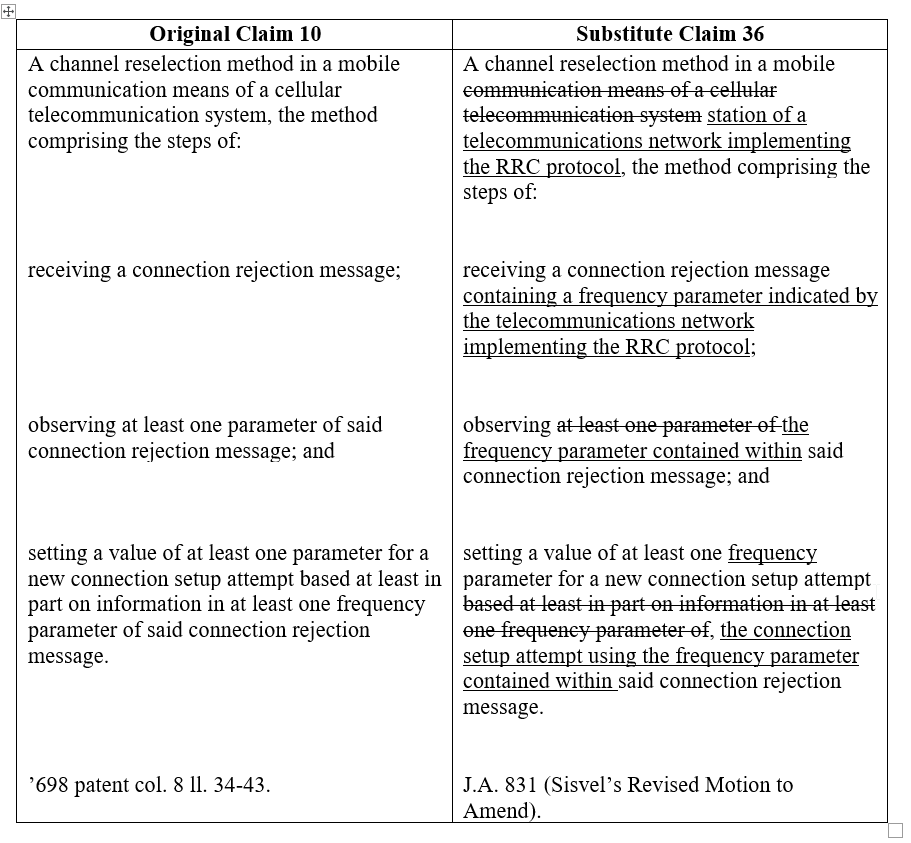
The amendments to the preamble and the first two limitations result in a more limited claim than original claim 10. However, the PTAB and Federal Circuit held the third limitation “setting the value” to be broader than the original limitation. The original claim required that the value of at least one parameter be based at least in part on information in the at least one frequency parameter. The amended claim required the “use of the frequency parameter” which was interpreted as being broader in scope than the “based on” language of the original claim 10. Using the information was broader in scope than the old language “based at least in part on information in at least one frequency parameter.” “Using the parameter” was broader than basing it on the parameter. The Federal Circuit used the example of using a value V and multiplied by X and then dividing by X where the value X was used to calculate V, but the value V is not based on X. Thus, while proposed claim 36 is narrower overall than claim 10, the last limitation is broader. It is possible for infringement of claim 36 to exist where none existed for claim 10 since a device only “using the frequency parameter” of claim 36 would not infringe claim 10 but would infringe proposed claim 36. The amendment process does not permit a patentee to broaden any aspect of a claim even though the overall the claim is narrower, see Hockerson-Halberstadt, 183 F.3d at 1374 see also 37 C.F.R. § 1.175(b) (“A claim is a broadened claim if the claim is broadened in any respect.”)<... Read more
Federal Circuit Reiterates What Constitutes A Motivation to Combine, A Reasonable Expectation of Success, and Unexpected Results in New Chemical Compounds
August 30, 2023
In Sun Pharmaceutical Industries, Inc. v. Incyte Corporation, on August 22, 2023, the Federal Circuit affirmed a Final Written Decision of the Patent and Trial Appeal Board (the Board) of an inter partes review (IPR) asserting the claims of U.S. Patent No. 9,249,149 (the ’149 patent) as obvious under 35 U.S.C. § 103. The central argument was whether Sun’s “octo-deuterated” ruxolitinib analog (CTP-543) and “tetra-deuterated” ruxolitinib analogs, arising from claim 7 of the ’149 patent, were obvious in light of the prior art references presented by Incyte (Rodgers, Shilling, and the Concert Backgrounder).
Claim 7 recited:
The compound of claim 1, in which the compound is selected from the group consisting of:
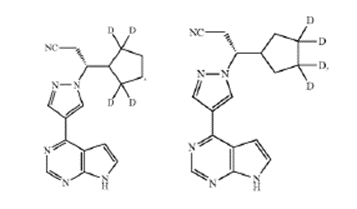
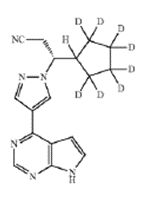
or a pharmaceutically acceptable salt of any of the foregoing.<... Read more
USPTO Issues Advance Notice of Proposed Rulemaking for America Invents Act (AIA) Proceedings Before the Patent Trial and Appeal Board
Attorney:
Richard D. Kelly
April 20, 2023
The USPTO today announced Advance Notice of Proposed Rulemaking for PTAB reforms regarding IPRs/PGRs. The proposal related to five areas:<... Read more
A Broad Outline of a Genus's Perimeter Is Insufficient For Written Description of the Members of the Genus
Attorney:
Marina I. Miller, Ph.D.
April 7, 2023
The Regents of the University of Minnesota (“Minnesota”) appealed from a final decision of the U.S. PTO Patent Trial and Appeal Board (“the Board”) holding that the claims of U.S. Patent 8,815,830 were unpatentable as anticipated. The Court of Appeals for the Federal Circuit (“the Court”) affirmed.<... Read more