Prosecution Disclaimer in a Member of a Patent Family Over the Prosecution History of Another Member of the Family
Attorney:
Richard D. Kelly
March 27, 2025
In Maquet Cardiovascular LLC. v. Abiomed Inc., Appeal No. 2023-2045, March 21, 2025, the Federal Circuit provided guidance as to when the prosecution history of one member of a patent family may act as an estoppel in the claim construction of another member of the family. At issue was the construction of claims 1 and 24 of U.S.P. 10,238,783 (‘783) in view of the prosecution histories of its parent application, U.S.P. 9,789,238 (‘238) and great-great-grandparent application U.S.P. 8,888,728 (‘728). The ‘783 patent was directed to blood pumps which could be placed in a patient’s vascular system without using a supplemental guide means. The guide was integrated into the apparatus.<... Read more
The CAFC Backs the FTC View on Listing Device Patents in the Orange Book
Attorney:
Richard D. Kelly
January 14, 2025
On December 20 the Federal Circuit in Teva Branded Pharmaceutical Products R&D, Inc. (Teva) v. Amneal Pharmaceuticals (Amneal) affirmed the district court’s decision that medical devices were not listable in the Orange Book and Teva had improperly listed patents in the Orange Book and required Teva to remove them.<... Read more
Myrbetriq® Patent Invalidated On Grounds Not Asserted by Any Party Violating Party Presentation Principle
Attorney:
Richard D. Kelly
October 2, 2024
In Astellas Pharma Inc. v. Sandoz Inc,, et al, following a five-day patent claims bench trial the court found asserted claims 5, 20, and 25 invalid under 35 U.S.C. § 101 even though Sandoz had not asserted 101 as defense at any time during the case. The parties had agreed to limit the issue to claims 5, 20, and 25 and the defense to invalidity under 35 U.S.C. § 112. Neither party had notice that patent eligibility was an issue. In making his ruling Judge Bataillon relied upon a statement in Astellas post-trial brief that the “inventive concept of the ’780 Patent was discovering the dissolution rate that would address the food effect and achieving it using previously known formulation technology.” Thus, because the claimed invention “reflects merely the discovery of the food-effect-resolving dissolution profile,” the district court deemed the asserted claims invalid as patent ineligible. Not only had the issue of patent eligibility not been raised by the defendant, but the claims were also to a specific composition. Recognizing the district court had gone on astray Sandoz moved the court to make additional findings of fact advising the court that the § 101 defense had not been raised as a defense and that the decision must be based on the language in the claims. The motion was denied.<... Read more
Finally, an ODP Decision in Favor of the Patentee
Attorney:
Richard D. Kelly
August 28, 2024
In Allergan USA, Inc v Sun Pharmaceutical Indus. Ltd, Appeal No. 2024-1061, August 13, 2024, the Federal Circuit reversed the district court’s holding that Allergan patent, U.S.P. 7,741,356 (‘356) was invalid for obviousness-type double patenting over USPs 11,007,179, 11,090,291, and 11,311,516. The relationship between the patents is shown below:
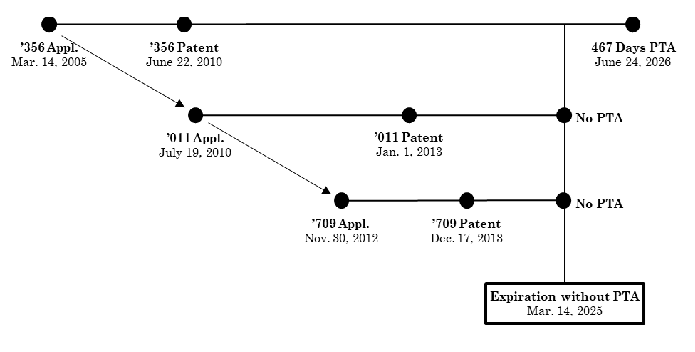
The ‘356 patent was entitled to significant PTA but because of the PTE it received, Allergan effectively disclaimed all but 487 days of the PTA. The total extension PTE plus PTA resulted in an expiration date of May 27, 2029, 15 years from the ‘356 issue date -- the maximum extension possible.<... Read more
Written Description of a Numerical Range
Attorney:
Richard D. Kelly
February 14, 2024
The Federal Circuit on February 9 decided another written description case involving a range not found in ipsis verbis in the patent specification in RAI Strategic Holdings Inc. v. Philip Morris Products S.A. RAI appealed from a PTAB holding in PGR2020-00071 that claims 10 and 27 of U.S.P. 10,492,542 lacked written description in the specification. Claims 27 is reproduced below; claim 10 is identical except it depends on claim 9:<... Read more
When does a drug label induce infringement under 35 U.S.C. § 271(e)(2) of a patent not claiming an indication or method of use?
Attorney:
Richard D. Kelly
January 23, 2024
In Corcept Therapeutics, Inc. v. Teva Pharmaceuticals U.S.A., Inc., C.A. 18-03632, December 29, 2023, the Chief Judge Bumb of the New Jersey District Court wrestled with the question of infringement under 35 U.S.C. 271(e)(2) where the drug labels of the generic and ethical drug were identical in the relevant parts.<... Read more
USPTO Releases Examiner Guidance for The Amgen Enablement Decision
Attorney:
Richard D. Kelly
January 10, 2024
The USPTO today published its guidance to the examiners on the impact of the Amgen v. Sanofi,143 S. Ct. 1243 (2023), on USPTO practice. The Guidance is basically steady as she goes but with the caveat that the enablement requirement and the Wands factors (In re Wands, 858 F.2d 731, 737 (Fed. Cir. 1988)) apply across all technologies, noting the Court’s reliance on cases involving the telegraph, incandescent lamp filaments and wood glue. The Guidance also noted that the post-Amgen Federal Circuit decisions had reaffirmed the continued validity of the Wands factors as consistent with Amgen, citingMedytox, 71 F.4th at 998- 999, Baxalta Inc, v. Genentech, Inc., 81 F.4th 1362 (Fed. Cir. 2023), and In re Starrett, 2023 WL 3881360 (Fed. Cir. 2023) (non-precedential).<... Read more
In Patent Eligibility It's the Claim That Is the Name of the Game*
Attorney:
Richard D. Kelly
December 18, 2023
Recently Judge Connolly, Chief Judge of the District Court of Delaware had an opportunity of deciding three motions for summary judgment filed by CareDX in its litigation with Natera, Natera, Inc. v. CareDX, Inc, Dist. DE, CA 20-38, that one patent was invalid as being directed to patent ineligible subject matter. The patents are U.S. Patent Nos. 10,597,724 (‘724), 10,655,180 (‘180), and 11,111,544 (‘544). The ‘724 and ‘180 patents are directed to methods of observing DNA in samples taken from patients. The ‘544 patent is directed to a method of “preparing a preparation of amplified DNA” from the sample of an individual to observe the DNA of a second individual in the sample. The Court found that one claim in each patent was representative of all claims in the respective patent. These representative claims are:<... Read more
Generics Beat the Tax Man
Attorney:
Richard D. Kelly
November 27, 2023
Recently Mylan in Mylan, Inc. v. Comm’r of Internal Revenue, 76 F4th 230 (3rd Cir. 2023), beat the tax man. Mylan tried to deduct its ANDA litigation expenses as ordinary and necessary business expenses which are an immediate deduction, while the Internal Revenue Service (IRS) claimed the expenses should be capitalized which is not as valuable. The IRS asserted that the expenses should be capitalized, under 26 U.S.C. § 263 and the associated regulations, similar to money paid to acquire approvals from the Food and Drug Administration (“FDA”). Mylan at 243. The Court rejected the IRS’ argument observing “ultimate FDA approval is never decided by the outcome of patent litigation under [35 U.S.C.] § 271(e)(2), even if it is delayed by such litigation.” Mylan at 244. The Court concluded “it makes no difference in deciding the question of deductibility whether the patent litigation expenses are incurred by the patentee or the alleged infringer. Nor does it matter that the deductibility question arises in the context of an ANDA suit.” Mylan at 239-40.<... Read more
Sisvel – PTAB Guidance On A Proposed Amendment In An IPR Is Not Binding On The PTAB
Attorney:
Richard D. Kelly
November 20, 2023
In Sisvel Int’l S.A. v. Sierra Wireless, Inc., Appeal nos. 2022-1387 and 2022-1492, (Fed. Cir. 2023) the Court considered this issue of when was a claim broadened in a post grant proceeding. Sisvel’s patent U.S. 7,433,698 (‘698) was involved in two IPRs, IPR 2020-01070 and IPR2020-01071 where it attempted unsuccessfully to amend its claim 10. Sisvel received preliminary guidance from the PTAB on its original motion to amend claim 10 which then led it to file a revised motion to amend. Sisvel’s revised motion proposed these amendments:
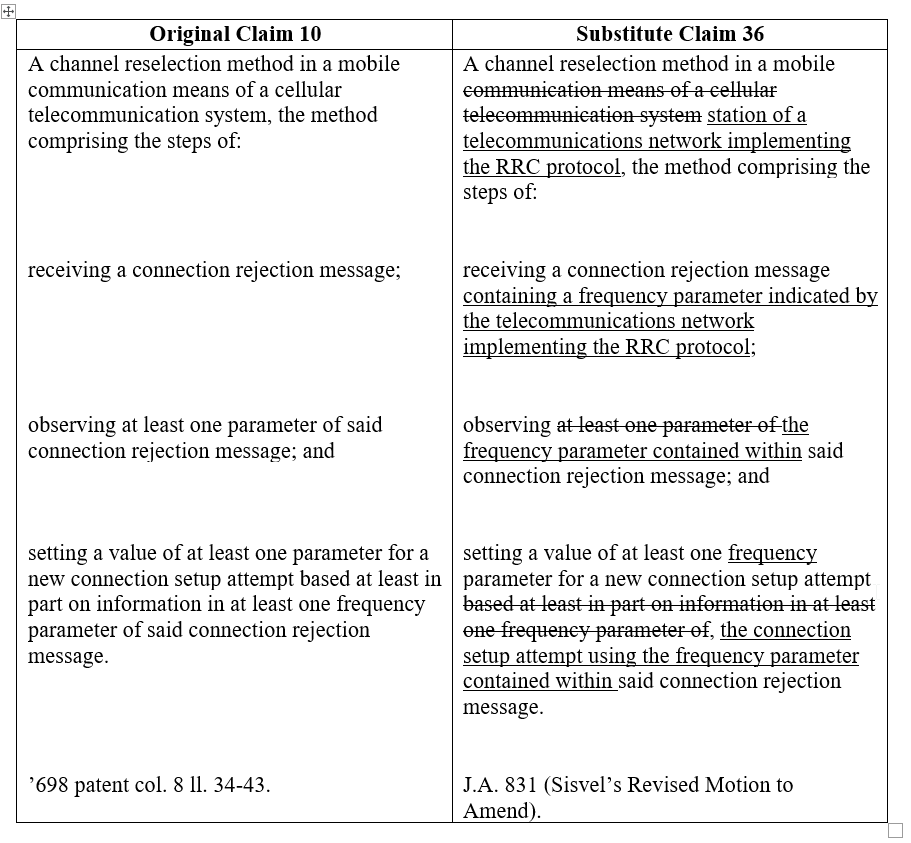
The amendments to the preamble and the first two limitations result in a more limited claim than original claim 10. However, the PTAB and Federal Circuit held the third limitation “setting the value” to be broader than the original limitation. The original claim required that the value of at least one parameter be based at least in part on information in the at least one frequency parameter. The amended claim required the “use of the frequency parameter” which was interpreted as being broader in scope than the “based on” language of the original claim 10. Using the information was broader in scope than the old language “based at least in part on information in at least one frequency parameter.” “Using the parameter” was broader than basing it on the parameter. The Federal Circuit used the example of using a value V and multiplied by X and then dividing by X where the value X was used to calculate V, but the value V is not based on X. Thus, while proposed claim 36 is narrower overall than claim 10, the last limitation is broader. It is possible for infringement of claim 36 to exist where none existed for claim 10 since a device only “using the frequency parameter” of claim 36 would not infringe claim 10 but would infringe proposed claim 36. The amendment process does not permit a patentee to broaden any aspect of a claim even though the overall the claim is narrower, see Hockerson-Halberstadt, 183 F.3d at 1374 see also 37 C.F.R. § 1.175(b) (“A claim is a broadened claim if the claim is broadened in any respect.”)<... Read more