Missing Feature Overcomes Obviousness but Cautions Succinct Claiming and Argument
October 7, 2024
On September 25, 2024, in Ex parte Sim, the Patent Trial and Appeal Board (PTAB) overturned the obviousness position of primary examiner, John Chu, supported by Supervisory Patent Examiners Mark Huff and Christine Tierney, in US Appl. No. 16/176,245 (Appeal 2023-003254, Technology Center 1700). Ex parte Sim involved a disagreement on the meaning of recitation regarding a copolymer structure.
The base claim under appeal in Ex parte Sim recited:
[a]n underlayer coating composition, comprising:
a polymer comprising:
repeat units derived from a monomer represented by Chemical Formula (1):
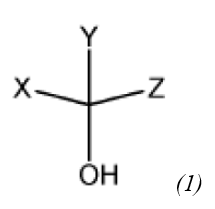
wherein,
X is a linear or branched C1 to C10 hydrocarbon group, a C1 to C10 alkoxycarbonyl group, a carboxylic acid group, or a linear or branched C1 to C10 hydroxyalkyl group optionally substituted with a C1 to C5 alkoxycarbonyl group or a C1 to C5 substituted alkoxy group;
Y is a hydrogen, a linear or branched C1 to C10 hydrocarbon group, a C1 to C10 alkoxycarbonyl group, a carboxylic acid group, or a linear or branched C1 to C10 hydroxyalkyl group optionally substituted with a C1 to C5 alkoxycarbonyl group or a C1 to C5 alkoxy group; and
Z is a linear or branched C1 to C10 hydrocarbon group, a C1 to C10 alkoxycarbonyl group, a carboxylic acid group, or a linear or branched C1 to C10 hydroxyalkyl group optionally substituted with a C1 to C5 alkoxycarbonyl group or a C1 to C5 alkoxy group,
wherein each of the C1 to C10 hydrocarbon group, the C1 to C10 alkoxycarbonyl group, and the C1 to C10 hydroxyalkylgroup is optionally substituted with at least one selected from the group consisting of fluorine, chlorine, bromine, iodine, a hydroxyl group, a thiol group, a carboxylic acid group, a C1 to C5 alkyl group, a C3 to C8 cycloalkyl group, a C2 to C5 alkenyl group, a C1 to C5 alkoxy group, a C2 to C5 alkenoxy group, a C6 to C10 aryl group, a C6 to C10 aryloxy group, a C7 to C10 alkylaryl group, and a C7 to C10 alkylaryloxy group,
provided that at least one selected from X, Y, and Z is:
a linear or branched C1 to C10 hydrocarbon group substituted with at least one selected from the group consisting of a hydroxyl group, a thiol group, a carboxylic acid group, a C2 to C5 alkenyl group, a C1 to C5 alkoxy group, a C2 to C5 alkenoxy group, a C6 to C10 aryloxy group, and a C7 to C10 alkylaryloxy group,
a C1 to C10 alkoxycarbonyl group,
a carboxylic acid group, or
a linear or branched C1 to C10 hydroxyalkyl group optionally substituted with a C1 to C5 alkoxycarbonyl group or a C1 to C5 alkoxy group, and
repeat units derived from a monomer represented by Chemical Formula (3):
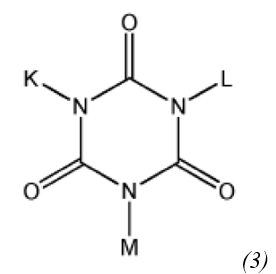
wherein, K, L, and M are each independently a linear or branched C1 to C10 hydrocarbon group, a C1 to C10 alkoxycarbonyl group, a C1 to C10 alkanoyloxy group, each of which is optionally substituted with a carboxylic acid group, or a linear or branched C1 to C10 hydroxyalkyl group optionally substituted with a Cl to C5 alkoxycarbonyl group or a Cl to C5 substituted alkoxy group,
wherein each of the C1 to C 10 hydrocarbon group, the C1 to C10 alkoxycarbonyl group, the C1 to C10 alkanoyloxy group, and the C1 to C10 hydroxyalkyl group is optionally substituted with at least one selected from the group consisting of fluorine, chlorine, bromine, iodine, C1 to C5 alkyl, C3 to C8 cycloalkyl, C2 to C5 alkenyl, Cl to C5 alkoxy, C2 to C5 alkenoxy, C6 to C10 aryl, C6 to C10 aryloxy, C7 to C10 alkylaryl, and C7 to C10 alkylaryloxy, and
4 to 15 wt% of a photoacid generator comprising an iodonium cation, based on total solid content of the underlayer coating composition.
The rejection of the above claim was based upon repeating unit structure (4-2) in the primary reference as follows -- using the annotating from the examiner beyond the correction of “2” to “3” in formula (4-2):
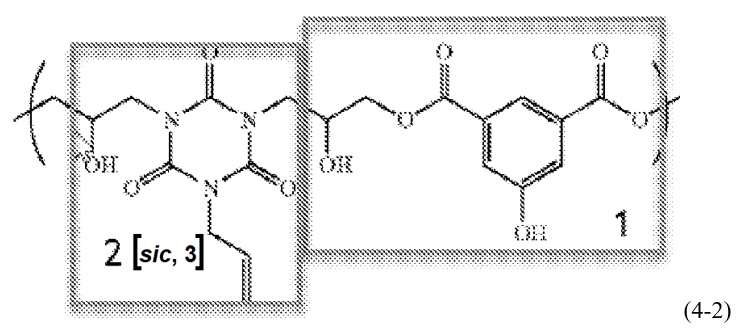
In the rejection, the examiner relied upon the central hydroxyl group (not individually boxed, but on the inside of the box on the right, on the left side of that box) for the “repeat units derived from a monomer represented by Chemical Formula (1).”
The appellant argued that the prior art described that (emphasis by the appellant):
[t]he polymer or the polymer precursor including the repeating unit of Formula (3) in which Q2 is an oxygen atom and T1 is a divalent linking group gives a polyether compound by a reaction of aromatic compounds having a hydroxy group to each other. The polymer or the polymer precursor in which Q2 is an oxycarbonyl group and T1 is a divalent linking group gives a polyester compound. The polymer or the polymer precursor in which Q2 is an oxygen atom or an oxycarbonyl group and T1 is a compound including an alkylene group substituted with a hydroxy group is a reaction product of a compound including a hydroxy group or a carboxy group and an epoxy compound. In this case, one of the aromatic compound and the compound having a divalent linking group of T1 is the compound including a hydroxy group or a carboxy group and the other is the epoxy compound.
The above description was in relation to the general structure (3):
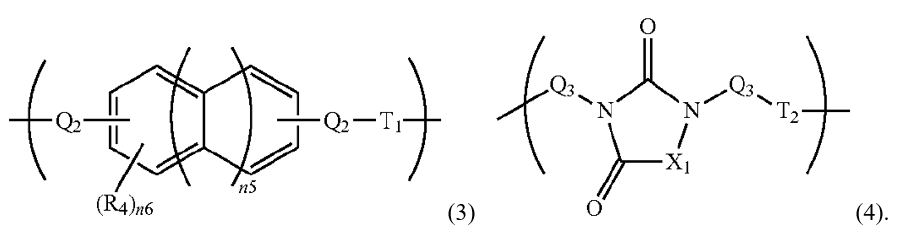
Regarding the prior art repeating unit of formula (4), the prior art described similarly that
[t]he polymer or the polymer precursor including the repeating unit of Formula (4) can form a polymer by a reaction of a hydrogen atom on a nitrogen atom with a diepoxy compound. Alternatively, a glycidyl group on a nitrogen atom is reacted with a dialcohol compound or a dicarboxylic acid compound to form a polymer or a polymer precursor.
The appellant argued that to conform to Formula (3) and (4), the prior art monomer required a reactive hydroxy group or a carboxy group, and the second prior art monomer required a reactive epoxy group. The appellant argued that the claimed substituent groups X, Y, and Z in the monomer of formula (1) did not include an epoxy group and were not optionally substituted with an epoxy group, nor K, L, M in the monomer of formula (3):
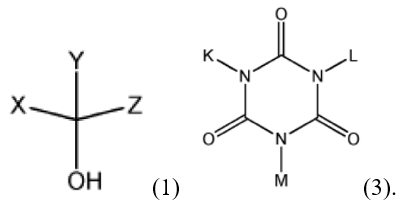
Thus, the appellant argued, unlike Sakamoto’s polymers, prepared by reacting at least one monomer including a reactive epoxy group, the claimed polymer does not include repeat units derived from a monomer having a reactive epoxy group.
The appellant provided an annotated comparison of the structure of the claimed monomers of formula (1) and (2) in the appeal brief, relative to the structure in the primary reference of formula (4-2), to illustrated the distinction argued by the appellant:
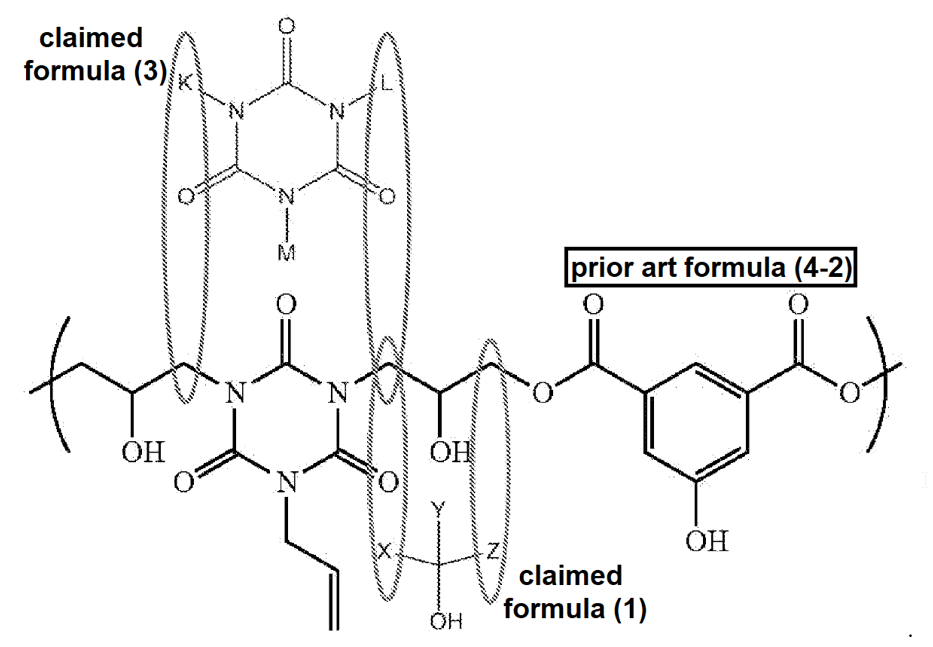
The appellant argued that the office action required that “X” in formula (1) and “L” in formula (3) is the same carbon atom of the polymer structure, i.e., that “L” requires at least one carbon atom, and may not be a hydrogen atom [sic, may not be a bonding point], and “X” requires at least one carbon atom, the “proposed reactivity to form the polymer of Formula (4-2) in [the prior art] is not possible based on the monomer structures of Chemical Formula (1) and Chemical Formula (3).”
The brief PTAB opinion reversed the examiner’s position with little extra detail, stating that the obviousness finding was unsustainable because “Formula (4-2) lacks a substituent group, for example at least one carbon atom between box 1… and box 2.”
The present case is therefore essentially an example of non-obviousness via a “missing element” or failure to disclose the claimed structure. However, the claim language and style of argument in this case is illustrative of difficulties that can arise in prosecution with claim language for polymers. As is frequently the case in polymer chemistry, the polymer can be easier to describe based upon the monomers than on the ultimate polymer structure, since polymers are ordinarily distributions of molecules of different chain lengths, often having different individual compositions and/or structural features. However, the repeating units or monomers are typically more easily definable.
Although it did not occur in prosecution in Ex parte Sim, the USPTO can reject the phrase, “derived from,” as indefinite under 35 USC § 112(b), and require applicants to define the term. The appellant in Ex parte Sim avoided direct argument on the meaning of “derived from,” but will be bound nonetheless by prosecution history estoppel to the definitions argued to overcome the prior art.
The appellant argued that the prior art required a reaction between a glycidyl group and a reactive hydrogen group. One analog of this is shown below, wherein the glycidyl group (methylene epoxide) is attached to nitrogens of the cyanurate (six-membered alternating nitrogen and carbonyl ring) and react with a succinate or 3,5-dicarboxyphenol:
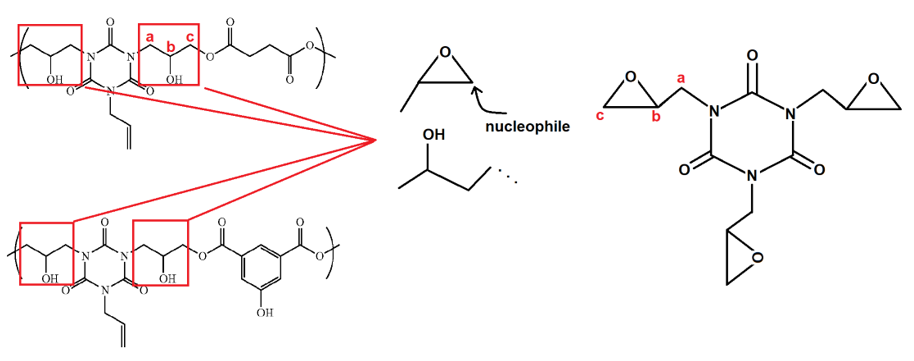
Although the appellant in this case argued that the monomer had certain substituents and that the Markush language limited substitution on the substituents, i.e., that the substituents to the monomers did not include epoxides, this should not have been dispositive. Because the claim ultimately covers a “polymer comprising” the particular “repeating units derived from” particular monomers, i.e., product-by-process language, the ultimate structure of the polymer was dispositive in Ex parte Sim. Fortunately for the appellant, it was able to be shown that the hydroxyl group in the prior art structure (labelled as “a,” “b,” and “c” above) equated to the monomer / repeating unit of formula (1):
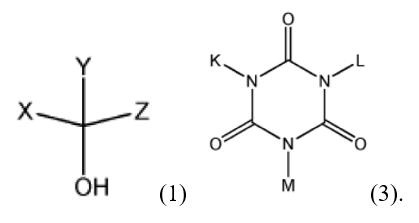
X, Y, and Z of formula (1), ignoring rotation, require a linear or branched C1 to C10 hydrocarbon group, a C1 to C10 alkoxycarbonyl group, a carboxylic acid group, or a linear or branched C1 to C10 hydroxyalkyl group, with Y optionally further being H. In the monomer / repeating unit of structure (3) above, K, L, and M were each a linear or branched C1 to C10 hydrocarbon group, a C1 to C10 alkoxycarbonyl group, a C1 to C10 (substituted) alkanoyloxy group. Because of these substituent recitations, the base claim recited a polymer having at least one additional carbon α to the position, “a,” which, in the prior art, was only a methylene (C1) portion of a glycidyl group (whether or not a glycidyl group was involved). That is, the additional carbon claimed, which was part of a substituent not displaced during the polymerization or formation of the macromonomer, distinguished the claims over the cited art, rather than the origin of the single carbon.
The appellant in Ex parte Sim only required the second monomer unit in the base claim after the second (appealed) final rejection, by taking up a dependent claim. Only at that point did the arguments regarding the extra atom become effective. However, rather than succinctly pointing out the distinction between the base claim and the cited art in the response to the final rejection, or interviewing the case with the examiner, the appellant submitted lengthy arguments and citations to case law without clearly pointing out the structural difference.
Thus, Ex parte Sim offers the incite that applicants may avoid appeal where a structural distinction exists and is not understood by the examiner, by interviewing the case with the examiner. Moreover, applicants should bear in mind that the time for evaluation of arguments (and interviews) at the USPTO is quite limited, and presenting succinct arguments can often be more effective.