PTAB Reiterates Prior Art Must Describe a Claimed Range with Sufficient Specificity to Support an Anticipation Rejection
April 30, 2024
On May 2, 2023, the Patent Trial and Appeal Board (PTAB) overturned a rejection of a claim directed to microparticles containing leuprolide and a biodegradable polymer, and a method for producing the same as anticipated and obvious (Appeal 2024-000508). The claim at issue of the application US 17/832,229 (the ‘229 Application) is directed towards:
Claim 1. Microparticles containing leuprolide and a biodegradable polymer and having an average diameter of 40 to 100 µm and a value of 0.5 to 2 as determined by the following Equation 1:
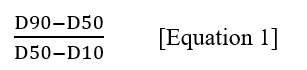
wherein D10 is a particle diameter corresponding to 10% cumulative (from 0 to 100%) undersize particle size distribution,
D50 is a particle diameter corresponding to 50% cumulative (from 0 to 100%) undersize particle distribution,
wherein the leuprolide and the biodegradable polymer are contained in a weight ratio of 1:2 to 1:10.
Of note, is the Examiner’s rejection under 35 U.S.C. § 102(a)(1). The Examiner asserted that the claimed microparticles were anticipated by Thanoo et al., WO 01/10414 A1 (“Thanoo”), stating Thanoo discloses a slow-release microsphere having an average particle size between 10-40 µm, including microparticles comprising leuprolide and biodegradable polymer (Appeal Decision p. 5).
The Board centered its analysis on whether the Examiner’s anticipation rejection based on the overlapping particle size range was proper. During prosecution, the Examiner interpreted the disclosure of Thanoo to disclose an average particle size between 10 to 40 µm because Thanoo recited “50% of the particle diameter is 48.4 µm or less, and 80% has a diameter between 23 to 69.7 µm” (Appeal Decision p. 5).
Appellant argued the claimed average particle size range was not anticipated by Thanoo, and that the claimed range was critical for the effective administration and sustained release of leuprolide from microparticles (Appeal Decision p. 6). The Board agreed with Appellant that the Examiner’s interpretation of Thanoo disclosing an average particle size of 48.4 µm was incorrect, asserting that the teaching of Thanoo related to particle size distribution and was not a disclosure of a particular average particle size.
The Board further agreed with Appellant, asserting that anticipation of a claimed range by an overlapping range disclosed in the prior art requires that the overlap in the prior art “describes the entire claimed range with sufficient specificity” such that one of ordinary skill in the art would understand there is no reasonable difference in how the invention operates over the ranges. Therefore, because the prior art did not disclose the claimed range with sufficient specificity for one of ordinary skill in the art to have understood a particle size outside of the claimed range would result in the claimed microparticles and Appellant demonstrated the claimed particle size range was critical for the effective administration and sustained release of leuprolide from microparticles, the Board reversed the rejection.
It is worth noting that the basis of this argument varies depending on whether the prior art discloses a point within the claimed range or discloses its own range that overlaps with the claimed range. If the prior art discloses a point within the claimed range, the prior art anticipates the claim. On the other hand, as in the case of the ‘229 Application, if the prior art discloses an overlapping range, the prior art anticipates the claimed range if it describes the claimed range with sufficient specificity such that one of ordinary skill in the art would conclude there is no reasonable difference in how the invention operates over the ranges. This decision serves as a reminder for practitioners that, when faced with an anticipation rejection based on an overlapping range, a showing of criticality of the claimed range may prove successful.