PTAB Restates That Not All Combinations of Molecular Modifications are Obvious
April 26, 2021
In a Patent Trial and Appeal Board (PTAB) decision issued on April 16, 2021, in Ex parte Bhalla, Luthra, Reid I, and Levason (Appeal 2021-001535, USSN 14/373,413), the PTAB was presented with the issue of the obviousness of an imaging agent comprising an 18F-labelled compound of a formula below, with the relevant moieties indicated. The main claim covered a moderate genus of compounds[1], and was rejected over a combination of three references.
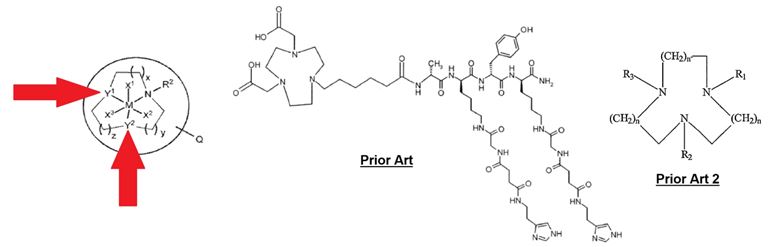
The PTAB noted that the rejection of the main claims was premised upon a primary reference which failed to describe the ligands at position Y1 and Y2, which is typified by the center structure above. The PTAB reported that the examiner sought out a secondary reference, describing anticancer treatment utility – rather than directly imaging as in the primary reference and the claims – and at least one overlapping metal, with groups covered by the claims at position Y1 and Y2. The Examiner further relied upon a tertiary, and potentially quaternary, reference disclosing that 18F-labelled compounds similar to the secondary reference are useful as imaging agents, and provided advantages in certain 5 or 6-membered (crown) rings in fluoride binding.
The PTAB considered the examiner’s rationale that substituting acetate pendent arms with CH3 and optionally substituting Al3+ with Ga3+ , would have been expected to provide functionally equivalent ligands and complex thereof suitable for in vivo PET imaging and/or
advantageously enable high labeling efficiency since CH3 cannot form 5- or 6-membered ring with Al3+.
However, while acknowledging that KSR Int’l Co. v. Teleflex Inc., 550 U.S. 398 (2007) emphasized “an expansive and flexible approach” when evaluating claims for obviousness, the PTAB noted that, in cases involving claims to new chemical compounds, “it remains necessary to identify some reason that would have led a chemist to modify a known compound in a particular manner to establish prima facie obviousness of a new claimed compound.” Takeda Chem. Indus., Ltd. v. Alphapharm Pty., Ltd., 492 F.3d 1350, 1357 (Fed. Cir. 2007).
The PTAB accepted the applicant’s argument that the primary reference actually taught the use of carboxylate or amine functional groups and expressly disclosed hydrophilic chelating compounds. The PTAB noted that the examiner was using a secondary reference with a cancer treatment utility and that the particular embodiments closest to the claims were indicated in the secondary reference to lack sufficient water solubility for the utility of the secondary reference. The PTAB considered and rejected the examiner’s arguments on the tertiary reference remedying the combination, because the tertiary reference, on the whole, taught hydrophilic moieties, i.e., acetates – unlike the lipophilic moieties claimed, on the amines.
The PTAB therefore overturned the examiner’s finding of obviousness for a lack of a reason that would have led a chemist to modify a known compound in a particular manner to establish prima facie obviousness of a new claimed compound.
This case reminds us not only that the USPTO is not entirely free to reconstruct claims out of a patchwork of prior art based primarily on hindsight, but also that the most effective argument in favor of non-obviousness in the US is based on non-combinability, incompatibility, and/or technical conflict in the prior art combination (which relates to the prima facie case of obviousness), rather than an argument based on the positive effect of the invention (which generally relates to rebuttal and secondary considerations).
[1] For context, in the molecule claimed, Y1 and Y2 are independently O or NR1, where R1 is C1-3 alkyl or -CH2-Ar1, wherein Ar1 is C5-12 aryl or C3-12 heteroaryl; X1, X2 and X3 are independently 19F or 18F, with the proviso that at least one of X1, X2 and X3 is 18F and at least one of the remaining X1, X2, and X3 is 19F; M is Al3+, Ga3+, In3+, Se3+, Y3+, Ho3+, Er3+, Tm3+, Yb3+ or Lu3+; x, y and z are 1; R2 is R1 or Q; Q is -L-[BTM], and may be present or absent; when present it is either R2 or is attached at one of the carbon atoms of the -(CH2)(CH2)x-, -(CH2)(CH2)y- or -(CH2)(CH2)z- groups, where BTM is a biological targeting moiety; wherein L is a synthetic linker group of formula -(A)m- wherein each A is independently -CR32-, -CR3=CR3-, -C=C-, -CR32CO2-, -CO2CR32-, -NR3CO-, -CONR3-, -CR3=N-O-, -NR3(C=O)NR3-, -NR3(C=S)NR3-, -SO2NR3-, -NR3SO2, -CR32OCR32,-CR32SCR32, -CR32NR3CR32-, a C4-8 cycloheteroalkylene group, a C4-8 cycloalkylene group, -Ar2-, -NR3-Ar2-, -O-Ar2-, -Ar2-CO)-, an amino acid, a sugar or a monodisperse polyethyleneglycol (PEG) building block, wherein each R3 is independently chosen from H, C1-4 alkyl, C2-4 alkenyl, C2-4 alkynyl, C1-4 alkoxyalkyl or C1-4 hydroxyalkyl; m is an integer of value 1 to 20; and each Ar2 is independently a C5-12 arylene group, or a C3-12 heteroarylene group.